Kawasaki disease
| Kawasaki disease | |
|---|---|
| Other names | Kawasaki syndrome,[1] mucocutaneous lymph node syndrome[2] |
 | |
| A medical illustration depicting Kawasaki disease. | |
| Specialty | Pediatrics, Rheumatology, Immunology |
| Symptoms | Fever > 5 days, large lymph nodes, rash, sore throat, diarrhea[1] |
| Complications | Coronary artery aneurysms[1] |
| Usual onset | < 5 years old[1] |
| Duration | ~ 3 weeks[1] |
| Causes | Unknown[1] |
| Risk factors | Age of < 5 years old |
| Diagnostic method | Based on symptoms, ultrasound of the heart[1] |
| Differential diagnosis | Scarlet fever, juvenile rheumatoid arthritis, paediatric multisystem inflammatory syndrome[3][1] |
| Medication | Oral Aspirin and intravenous immunoglobulin[1] |
| Prognosis | Mortality 0.2% with treatment[4] |
| Frequency | 8–124 per 100,000 people under five[5] |
| Named after | Tomisaku Kawasaki |
Kawasaki disease (also known as mucocutaneous lymph node syndrome) is a syndrome of unknown cause that results in a fever and mainly affects children under 5 years of age.[6] It is a form of vasculitis, in which medium-sized blood vessels become inflamed throughout the body.[1] The fever typically lasts for more than five days and is not affected by usual medications.[1] Other common symptoms include large lymph nodes in the neck, a rash in the genital area, lips, palms, or soles of the feet, and red eyes.[1] Within three weeks of the onset, the skin from the hands and feet may peel, after which recovery typically occurs.[1] The disease is the leading cause of acquired heart disease in children in developed countries, which include the formation of coronary artery aneurysms and myocarditis.[1][7]
While the specific cause is unknown, it is thought to result from an excessive immune response to particular infections in children who are genetically predisposed to those infections.[6] It is not an infectious disease, that is, it does not spread between people.[8] Diagnosis is usually based on a person's signs and symptoms.[1] Other tests such as an ultrasound of the heart and blood tests may support the diagnosis.[1] Diagnosis must take into account many other conditions that may present similar features, including scarlet fever and juvenile rheumatoid arthritis.[9] Multisystem inflammatory syndrome in children, a "Kawasaki-like" disease associated with COVID-19,[10] appears to have distinct features.[11][12]
Typically, initial treatment of Kawasaki disease consists of high doses of aspirin and immunoglobulin.[1] Usually, with treatment, fever resolves within 24 hours and full recovery occurs.[1] If the coronary arteries are involved, ongoing treatment or surgery may occasionally be required.[1] Without treatment, coronary artery aneurysms occur in up to 25% and about 1% die.[4][13] With treatment, the risk of death is reduced to 0.17%.[13] People who have had coronary artery aneurysms after Kawasaki disease require lifelong cardiological monitoring by specialized teams.[14]
Kawasaki disease is rare.[1] It affects between 8 and 67 per 100,000 people under the age of five except in Japan, where it affects 124 per 100,000.[5] Boys are more commonly affected than girls.[1] The disorder is named after Japanese pediatrician Tomisaku Kawasaki, who first described it in 1967.[5][15]
Signs and symptoms
[edit]
Kawasaki disease often begins with a high and persistent fever that is not very responsive to normal treatment with paracetamol (acetaminophen) or ibuprofen.[16][17] This is the most prominent symptom of Kawasaki disease, and is a characteristic sign that the disease is in its acute phase; the fever normally presents as a high (above 39–40 °C) and remittent, and is followed by extreme irritability.[17][18] Recently, it is reported to be present in patients with atypical or incomplete Kawasaki disease;[19][20] nevertheless, it is not present in 100% of cases.[21]
The first day of fever is considered the first day of the illness,[16] and its duration is typically one to two weeks; in the absence of treatment, it may extend for three to four weeks.[4] Prolonged fever is associated with a higher incidence of cardiac involvement.[22] It responds partially to antipyretic drugs and does not cease with the introduction of antibiotics.[4] However, when appropriate therapy is started – intravenous immunoglobulin and aspirin – the fever subsides after two days.[23]
Bilateral conjunctival inflammation has been reported to be the most common symptom after fever.[24][25] It typically involves the bulbar conjunctivae, is not accompanied by suppuration, and is not painful.[26] This usually begins shortly after the onset of fever during the acute stage of the disease.[16] Anterior uveitis may be present under slit-lamp examination.[27][28] Iritis can occur, too.[29] Keratic precipitates are another eye manifestation (detectable by a slit lamp, but are usually too small to be seen by the unaided eye).[16][30]
Kawasaki disease also presents with a set of mouth symptoms, the most characteristic of which are a red tongue, swollen lips with vertical cracking, and bleeding.[31] The mucosa of the mouth and throat may be bright red, and the tongue may have a typical "strawberry tongue" appearance (marked redness with prominent gustative papillae).[4][32] These mouth symptoms are caused by necrotizing microvasculitis with fibrinoid necrosis.[31]
Cervical lymphadenopathy is seen in 50% to 75% of children, whereas the other features are estimated to occur in 90%,[16][24] but sometimes it can be the dominant presenting symptom.[30][33] According to the diagnostic criteria, at least one impaired lymph node ≥ 15 mm in diameter should be involved.[32] Affected lymph nodes are painless or minimally painful, nonfluctuant, and nonsuppurative; erythema of the neighboring skin may occur.[16] Children with fever and neck adenitis who do not respond to antibiotics should have Kawasaki disease considered as part of the differential diagnoses.[16]
| Less common manifestations | |
|---|---|
| System | Manifestations |
| GIT | Diarrhea, chest pain, abdominal pain, vomiting, liver dysfunction, pancreatitis, hydrops gallbladder,[34] parotitis,[24][35] cholangitis, intussusception, intestinal pseudo-obstruction, ascites, splenic infarction |
| MSS | Polyarthritis and arthralgia |
| CVS | Myocarditis, pericarditis, tachycardia,[32] valvular heart disease |
| GU | Urethritis, prostatitis, cystitis, priapism, interstitial nephritis, orchitis, nephrotic syndrome |
| CNS | Lethargy, semicoma,[24] aseptic meningitis, and sensorineural deafness |
| RS | Shortness of breath,[32] influenza-like illness, pleural effusion, atelectasis |
| Skin | Erythema and induration at BCG vaccination site, Beau's lines, and finger gangrene |
| Source: review,[32] table.[36] | |
In the acute phase of the disease, changes in the peripheral extremities can include erythema of the palms and soles, which is often striking with sharp demarcation[16] and often accompanied by painful, brawny edema of the dorsa of the hands or feet, so affected children frequently refuse to hold objects in their hands or to bear weight on their feet.[4][16] Later, during the convalescent or the subacute phase, desquamation of the fingers and toes usually begins in the periungual region within two to three weeks after the onset of fever and may extend to include the palms and soles.[37] Around 11% of children affected by the disease may continue skin-peeling for many years.[38] One to two months after the onset of fever, deep transverse grooves across the nails may develop (Beau's lines),[39] and occasionally nails are shed.[39]
The most common skin manifestation is a diffuse macular-papular erythematous rash, which is quite nonspecific.[40] The rash varies over time and is characteristically located on the trunk; it may further spread to involve the face, extremities, and perineum.[4] Many other forms of cutaneous lesions have been reported; they may include scarlatiniform, papular, urticariform, multiform-like erythema, and purpuric lesions; even micropustules were reported.[41][42] It can be polymorphic, not itchy, and normally observed up to the fifth day of fever.[43] However, it is never bullous or vesicular.[4]
In the acute stage of Kawasaki disease, systemic inflammatory changes are evident in many organs.[44] Joint pain (arthralgia) and swelling, frequently symmetrical, and arthritis can also occur.[24] Myocarditis,[45] diarrhea,[32] pericarditis, valvulitis, aseptic meningitis, pneumonitis, lymphadenitis, and hepatitis may be present and are manifested by the presence of inflammatory cells in the affected tissues.[44] If left untreated, some symptoms will eventually relent, but coronary artery aneurysms will not improve, resulting in a significant risk of death or disability due to myocardial infarction.[32] If treated quickly, this risk can be mostly avoided and the course of illness cut short.[46]
Other reported nonspecific symptoms include cough, rhinorrhea, sputum, vomiting, headache, and seizure.[24]
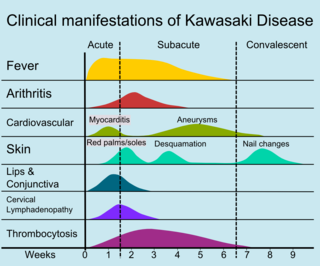
The course of the disease can be divided into three clinical phases.[48]
- The acute febrile phase, which usually lasts for one to two weeks, is characterized by fever, conjunctival injection, erythema of the oral mucosa, erythema and swelling of the hands and feet, rash, cervical adenopathy, aseptic meningitis, diarrhea, and hepatic dysfunction.[32] Myocarditis is common during this time, and a pericardial effusion may be present.[16] Coronary arteritis may be present, but aneurysms are generally not yet visible by echocardiography.
- The subacute phase begins when fever, rash, and lymphadenopathy resolve at about one to two weeks after the onset of fever, but irritability, anorexia, and conjunctival injection persist. Desquamation of the fingers and toes and thrombocytosis are seen during this stage, which generally lasts until about four weeks after the onset of fever. Coronary artery aneurysms usually develop during this time, and the risk for sudden death is highest.[16][49]
- The convalescent stage begins when all clinical signs of illness have disappeared, and continues until the sedimentation rate returns to normal, usually at six to eight weeks after the onset of illness.[32]
Adult onset of Kawasaki disease is rare.[50] The presentation differs between adults and children: in particular, it seems that adults more often have cervical lymphadenopathy, hepatitis, and arthralgia.[32][50]
Some children, especially young infants,[51] have atypical presentations without the classic set of symptoms.[48] Such presentations are associated with a higher risk of cardiac artery aneurysms.[16][52]
Cardiac
[edit]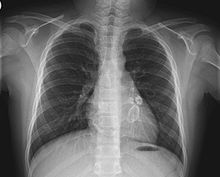
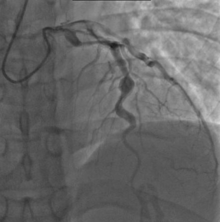
Heart complications are the most important aspect of Kawasaki disease, which is the leading cause of heart disease acquired in childhood in the United States and Japan.[32] In developed nations, it appears to have replaced acute rheumatic fever as the most common cause of acquired heart disease in children.[16] Coronary artery aneurysms occur as a sequela of the vasculitis in 20–25% of untreated children.[53] It is first detected at a mean of 10 days of illness and the peak frequency of coronary artery dilation or aneurysms occurs within four weeks of onset.[49] Aneurysms are classified into small (internal diameter of vessel wall <5 mm), medium (diameter ranging from 5–8 mm), and giant (diameter > 8 mm).[32] Saccular and fusiform aneurysms usually develop between 18 and 25 days after the onset of illness.[16]
Even when treated with high-dose IVIG regimens within the first 10 days of illness, 5% of children with Kawasaki disease develop at the least transient coronary artery dilation and 1% develop giant aneurysms.[54][55][56] Death can occur either due to myocardial infarction secondary to blood clot formation in a coronary artery aneurysm or to rupture of a large coronary artery aneurysm. Death is most common two to 12 weeks after the onset of illness.[16]
Many risk factors predicting coronary artery aneurysms have been identified,[22] including persistent fever after IVIG therapy,[57][58] low hemoglobin concentrations, low albumin concentrations, high white-blood-cell count, high band count, high CRP concentrations, male sex, and age less than one year.[59] Coronary artery lesions resulting from Kawasaki disease change dynamically with time.[4] Resolution one to two years after the onset of the disease has been observed in half of vessels with coronary aneurysms.[60][61] Narrowing of the coronary artery, which occurs as a result of the healing process of the vessel wall, often leads to significant obstruction of the blood vessel and the heart not receiving enough blood and oxygen.[60] This can eventually lead to heart muscle tissue death, i.e., myocardial infarction (MI).[60]
MI caused by thrombotic occlusion in an aneurysmal, stenotic, or both aneurysmal and stenotic coronary artery is the main cause of death from Kawasaki disease.[62] The highest risk of MI occurs in the first year after the onset of the disease.[62] MI in children presents with different symptoms from those in adults. The main symptoms were shock, unrest, vomiting, and abdominal pain; chest pain was most common in older children.[62] Most of these children had the attack occurring during sleep or at rest, and around one-third of attacks were asymptomatic.[16]
Valvular insufficiencies, particularly of mitral or tricuspid valves, are often observed in the acute phase of Kawasaki disease due to inflammation of the heart valve or inflammation of the heart muscle-induced myocardial dysfunction, regardless of coronary involvement.[60] These lesions mostly disappear with the resolution of acute illness,[63] but a very small group of the lesions persist and progress.[64] There is also late-onset aortic or mitral insufficiency caused by thickening or deformation of fibrosed valves, with the timing ranging from several months to years after the onset of Kawasaki disease.[65] Some of these lesions require valve replacement.[66]
Other
[edit]Other Kawasaki disease complications have been described, such as aneurysm of other arteries: aortic aneurysm,[67] with a higher number of reported cases involving the abdominal aorta,[68][69] axillary artery aneurysm,[70] brachiocephalic artery aneurysm,[71] aneurysm of iliac and femoral arteries, and renal artery aneurysm.[4][72] Other vascular complications can occur such as increased wall thickness and decreased distensibility of carotid arteries,[73] aorta,[74] and brachioradial artery.[75] This change in the vascular tone is secondary to endothelial dysfunction.[72] In addition, children with Kawasaki disease, with or without coronary artery complications, may have a more adverse cardiovascular risk profile later in life,[75] and may benefit from long term monitoring and prevention approaches to both detect cardiovascular disease early such as thrombosis or stenosis and prevent onset.[14] Examples of prevention approaches include lipid lowering medications, blood pressure medication, smoking cessation, and healthy active living.[14]
Gastrointestinal complications in Kawasaki disease are similar to those observed in Henoch–Schönlein purpura,[70] such as: intestinal obstruction,[76] colon swelling,[77] intestinal ischemia,[78] intestinal pseudo-obstruction,[79] and acute abdomen.[80]
Eye changes associated with the disease have been described since the 1980s, being found as uveitis, iridocyclitis, conjunctival hemorrhage,[81][82][83] optic neuritis,[70] amaurosis, and ocular artery obstruction.[84] It can also be found as necrotizing vasculitis, progressing into peripheral gangrene.[85]
The neurological complications per central nervous system lesions are increasingly reported.[86] The neurological complications found are meningoencephalitis,[87] subdural effusion,[88][89] cerebral hypoperfusion,[90] cerebral ischemia and infarct,[91] cerebellar infarction,[92] manifesting with seizures, chorea, hemiplegia, mental confusion, lethargy and coma,[70] or even a cerebral infarction with no neurological manifestations.[91] Other neurological complications from cranial nerve involvement are reported as ataxia,[70] facial palsy,[93] and sensorineural hearing loss.[94][95] Behavioral changes are thought to be caused by localised cerebral hypoperfusion,[90] can include attention deficits, learning deficits, emotional disorders (emotional lability, fear of night, and night terrors), and internalization problems (anxious, depressive or aggressive behavior).[96][97]
Causes
[edit]The specific cause of Kawasaki disease is unknown.[98][99][100][101] A plausible explanation is that it may be caused by an infection that triggers an inappropriate immunologic cascade in a small number of genetically predisposed children.[6][102] The pathogenesis is complex and incompletely understood.[103] Various explanations exist.[101] (See Classification)
Circumstantial evidence points to an infectious cause.[104] Since recurrences are unusual in Kawasaki disease, it is thought that the trigger is more likely to be represented by a single pathogen, rather than a range of viral or bacterial agents.[105] Various candidates have been implicated, including upper respiratory tract infection by some novel RNA virus.[6] Despite intensive search, no single pathogen has been identified.[103] There has been debate as to whether the infectious agent might be a superantigen (i.e. one commonly associated with excessive immune system activation).[101][106] Current consensus favors an excessive immunologic response to a conventional antigen which usually provides future protection.[6] Research points to an unidentified ubiquitous virus,[107] possibly one that enters through the respiratory tract.[108]
Seasonal trends in the appearance of new cases of Kawasaki disease have been linked to tropospheric wind patterns, which suggests wind-borne transport of something capable of triggering an immunologic cascade when inhaled by genetically susceptible children.[6] Winds blowing from central Asia correlate with numbers of new cases of Kawasaki disease in Japan, Hawaii, and San Diego.[109] These associations are themselves modulated by seasonal and interannual events in the El Niño–Southern Oscillation in winds and sea surface temperatures over the tropical eastern Pacific Ocean.[110] Efforts have been made to identify a possible pathogen in air-filters flown at altitude above Japan.[111] One source has been suggested in northeastern China.[6][112]
Genetics
[edit]Genetic susceptibility is suggested by increased incidence among children of Japanese descent around the world, and also among close and extended family members of affected people.[6] Genetic factors are also thought to influence development of coronary artery aneurysms and response to treatment.[113] The exact genetic contribution remains unknown.[114] Genome-wide association studies and studies of individual candidate genes have together helped identify specific single nucleotide polymorphisms (SNPs), mostly found in genes with immune regulatory functions.[113] The associated genes and their levels of expression appear to vary among different ethnic groups, both with Asian and non-Asian backgrounds.[115]
SNPs in FCGR2A, CASP3, BLK, ITPKC, CD40 and ORAI1 have all been linked to susceptibility, prognosis, and risk of developing coronary artery aneurysms.[115] Various other possible susceptibility genes have been proposed, including polymorphisms in the HLA region, but their significance is disputed.[114] Genetic susceptibility to Kawasaki disease appears complex.[116] Gene–gene interactions also seem to affect susceptibility and prognosis.[115] At an epigenetic level, altered DNA methylation has been proposed as an early mechanistic factor during the acute phase of the disease.[115]
Diagnosis
[edit]| Criteria for diagnosis |
|---|
| Fever of ≥5 days' duration associated with at least four† of these five changes |
| Bilateral nonsuppurative conjunctivitis |
| One or more changes of the mucous membranes of the upper respiratory tract, including throat redness, dry cracked lips, red lips, and "strawberry" tongue |
| One or more changes of the arms and legs, including redness, swelling, skin peeling around the nails, and generalized peeling |
| Polymorphous rash, primarily truncal |
| Large lymph nodes in the neck (>15 mm in size) |
| Disease cannot be explained by some other known disease process |
| †A diagnosis of Kawasaki disease can be made if fever and only three changes are present if coronary artery disease is documented by two-dimensional echocardiography or coronary angiography. |
| Source: Nelson's essentials of pediatrics,[117] Review[118] |
Since no specific laboratory test exists for Kawasaki disease, diagnosis must be based on clinical signs and symptoms, together with laboratory findings.[9] Timely diagnosis requires careful history-taking and thorough physical examination.[119] Establishing the diagnosis is difficult, especially early in the course of the illness, and frequently children are not diagnosed until they have seen several health-care providers. Many other serious illnesses can cause similar symptoms, and must be considered in the differential diagnosis, including scarlet fever, toxic shock syndrome, juvenile idiopathic arthritis, and childhood mercury poisoning (infantile acrodynia).[120]
Classically, five days of fever[121] plus four of five diagnostic criteria must be met to establish the diagnosis. The criteria are:[122]
- erythema of the lips or oral cavity or cracking of the lips
- rash on the trunk
- swelling or erythema of the hands or feet
- red eyes (conjunctival injection)
- swollen lymph node in the neck of at least 15 mm
Many children, especially infants, eventually diagnosed with Kawasaki disease, do not exhibit all of the above criteria. In fact, many experts now recommend treating for Kawasaki disease even if only three days of fever have passed and at least three diagnostic criteria are present, especially if other tests reveal abnormalities consistent with Kawasaki disease. In addition, the diagnosis can be made purely by the detection of coronary artery aneurysms in the proper clinical setting.[citation needed]
Investigations
[edit]A physical examination will demonstrate many of the features listed above.
Blood tests
- Complete blood count may reveal normocytic anemia and eventually thrombocytosis.
- Erythrocyte sedimentation rate will be elevated.
- C-reactive protein will be elevated.
- Liver function tests may show evidence of hepatic inflammation and low serum albumin levels.[123]
Other optional tests include:
- Electrocardiogram may show evidence of ventricular dysfunction or, occasionally, arrhythmia due to myocarditis.
- Echocardiogram may show subtle coronary artery changes or, later, true aneurysms.
- Ultrasound or computerized tomography may show hydrops (enlargement) of the gallbladder.
- Urinalysis may show white blood cells and protein in the urine (pyuria and proteinuria) without evidence of bacterial growth.
- Lumbar puncture may show evidence of aseptic meningitis.
- Angiography was historically used to detect coronary artery aneurysms, and remains the gold standard for their detection, but is rarely used today unless coronary artery aneurysms have already been detected by echocardiography.
Biopsy is rarely performed, as it is not necessary for diagnosis.[8]
Subtypes
[edit]Based on clinical findings, a diagnostic distinction may be made between the "classic" or "typical" presentation of Kawasaki disease and "incomplete" or "atypical" presentation of a "suspected" form of the disease.[6] Regarding incomplete/atypical presentation, American Heart Association guidelines state that Kawasaki disease "should be considered in the differential diagnosis of prolonged unexplained fever in childhood associated with any of the principal clinical features of the disease, and the diagnosis can be considered confirmed when coronary artery aneurysms are identified in such patients by echocardiography."[6]
A further distinction between incomplete and atypical subtypes may also be made in the presence of non-typical symptoms.[48]
Case definition
[edit]For study purposes, including vaccine safety monitoring, an international case definition has been proposed to categorize 'definite' (i.e. complete/incomplete), 'probable' and 'possible' cases of Kawasaki disease.[124]
Differential diagnosis
[edit]The broadness of the differential diagnosis is a challenge to timely diagnosis of Kawasaki disease.[9] Infectious and noninfectious conditions requiring consideration include: measles and other viral infections (e.g. adenovirus, enterovirus); staphylococcal and streptococcal toxin-mediated diseases such as scarlet fever and toxic shock syndrome; drug hypersensitivity reactions (including Stevens Johnson syndrome); systemic onset juvenile idiopathic arthritis; Rocky Mountain spotted fever or other rickettsial infections; and leptospirosis.[6] Infectious conditions that can mimic Kawasaki disease include periorbital cellulitis, peritonsillar abscess, retropharyngeal abscess, cervical lymphadenitis, parvovirus B19, mononucleosis, rheumatic fever, meningitis, staphylococcal scalded skin syndrome, toxic epidermal necrolysis, and Lyme disease.[8]
Kawasaki-like disease temporally associated with COVID-19
[edit]In 2020, reports of a Kawasaki-like disease following exposure to SARS-CoV-2, the virus responsible for COVID-19, emerged in the US and Europe.[125][10] The World Health Organization is examining possible links with COVID-19.[126] This emerging condition was named "paediatric multisystem inflammatory syndrome" by the Royal College of Paediatrics and Child Health,[3] and "multisystem inflammatory syndrome in children" by the Centers for Disease Control and Prevention.[127] Guidance for diagnosis and reporting of cases has been issued by these organizations.[3][126][127]
Several reported cases suggest that this Kawasaki-like multisystem inflammatory syndrome is not limited to children; there is the possibility of an analogous disease in adults, which has been termed MIS-A. Some suspected patients have presented with positive test results for SARS-CoV-2 and reports suggest intravenous immunoglobulin, anticoagulation, tocilizumab, plasmapheresis and steroids are potential treatments.[128][129][130]
Classification
[edit]Debate has occurred about whether Kawasaki disease should be viewed as a characteristic immune response to some infectious pathogen, as an autoimmune process, or as an autoinflammatory disease (i.e. involving innate rather than adaptive immune pathways).[101] Overall, immunological research suggests that Kawasaki disease is associated with a response to a conventional antigen (rather than a superantigen) that involves both activation of the innate immune system and also features of an adaptive immune response.[6][131] Identification of the exact nature of the immune process involved in Kawasaki disease could help guide research aimed at improving clinical management.[101]
Inflammation, or vasculitis, of the arteries and veins occurs throughout the body, usually caused by increased production of the cells of the immune system to a pathogen, or autoimmunity.[132] Systemic vasculitides may be classified according to the type of cells involved in the proliferation, as well as the specific type of tissue damage occurring within the vein or arterial walls.[132] Under this classification scheme for systemic vasculitis, Kawasaki disease is considered to be a necrotizing vasculitis (also called necrotizing angiitis), which may be identified histologically by the occurrence of necrosis (tissue death), fibrosis, and proliferation of cells associated with inflammation in the inner layer of the vascular wall.[132][133]
Other diseases involving necrotizing vasculitis include polyarteritis nodosa, granulomatosis with polyangiitis, Henoch–Schönlein purpura, and eosinophilic granulomatosis with polyangiitis.[132]
Kawasaki disease may be further classified as a medium-sized vessel vasculitis, affecting medium- and small-sized blood vessels,[44][134][135] such as the smaller cutaneous vasculature (veins and arteries in the skin) that range from 50 to 100 μm in diameter.[32][136] Kawasaki disease is also considered to be a primary childhood vasculitis, a disorder associated with vasculitis that mainly affects children under the age of 18.[118][137] A recent, consensus-based evaluation of vasculitides occurring primarily in children resulted in a classification scheme for these disorders, to distinguish them and suggest a more concrete set of diagnostic criteria for each.[118] Within this classification of childhood vasculitides, Kawasaki disease is, again, a predominantly medium-sized vessel vasculitis.[118]
It can also be classed as an autoimmune form of vasculitis.[4] It is not associated with anti-neutrophil cytoplasmic antibodies, unlike other vasculitic disorders associated with them (such as granulomatosis with polyangiitis, microscopic polyangiitis, and eosinophilic granulomatosis with polyangiitis).[132][138] This form of categorization is relevant for appropriate treatment.[139]
Treatment
[edit]Children with Kawasaki disease should be hospitalized and cared for by a physician who has experience with this disease. In an academic medical center, care is often shared between pediatric cardiology, pediatric rheumatology, and pediatric infectious disease specialists (although no specific infectious agent has yet been identified).[140] To prevent damage to coronary arteries, treatment should be started immediately following the diagnosis.[citation needed]
Intravenous immunoglobulin (IVIG) is the standard treatment for Kawasaki disease[141] and is administered in high doses with marked improvement usually noted within 24 hours. If the fever does not respond, an additional dose may be considered. In rare cases, a third dose may be given. IVIG is most useful within the first seven days of fever onset, to prevent coronary artery aneurysm. IVIG given within the first 10 days of the disease reduces the risk of damage to the coronary arteries in children, without serious adverse effects.[141] A 2023 systematic review and meta-analysis revealed that no prediction models of IVIG resistance in patients with Kawasaki disease could accurately distinguish the resistance.[142] The largest clinical study to date on treatment of resistant Kawasaki disease has shown that infliximab is more effective and safer than a second dose of IVIG in treating children with IVIG-resistant Kawasaki Disease, offering faster fever resolution and reduced hospitalization times without significant adverse events.[143]
Salicylate therapy, particularly aspirin, remains an important part of the treatment (though questioned by some)[144] but salicylates alone are not as effective as IVIG. There is limited evidence to indicate whether children should continue to receive salicylate as part of their treatment.[145] Aspirin therapy is started at high doses until the fever subsides, and then is continued at a low dose when the patient returns home, usually for two months to prevent blood clots from forming. Except for Kawasaki disease and a few other indications, aspirin is otherwise normally not recommended for children due to its association with Reye syndrome. Because children with Kawasaki disease will be taking aspirin for up to several months, vaccination against varicella and influenza is required, as these infections are most likely to cause Reye syndrome.[146]
High-dose aspirin is associated with anemia and does not confer benefit to disease outcomes.[147]
About 15-20% of children following the initial IVIG infusion show persistent or recurrent fever and are classified as IVIG-resistant. While the use of TNF alpha blockers (TNF-α) may reduce treatment resistance and the infusion reaction after treatment initiation, further research is needed.[148] Due to the potential involvement of the upregulated calcium-nuclear factor of activated T cells pathway in the development of the disease, a 2019 study found that the combination of ciclosporin and IVIG infusion can suppress coronary artery abnormalities. Further research is needed to determine which patients would respond best to this treatment.[149]
Corticosteroids have also been used,[150] especially when other treatments fail or symptoms recur, but in a randomized controlled trial, the addition of corticosteroid to immune globulin and aspirin did not improve outcome.[151] Additionally, corticosteroid use in the setting of Kawasaki disease is associated with increased risk of coronary artery aneurysm, so its use is generally contraindicated in this setting. In cases of Kawasaki disease refractory to IVIG, cyclophosphamide and plasma exchange have been investigated as possible treatments, with variable outcomes. However, a Cochrane review published in 2017 (updated in 2022) found that, in children, the use of corticosteroids in the acute phase of KD was associated with improved coronary artery abnormalities, shorter hospital stays, a decreased duration of clinical symptoms, and reduced inflammatory marker levels. Patient populations based in Asia, people with higher risk scores, and those receiving longer steroid treatment may have greater benefit from steroid use.[152]
Prognosis
[edit]With early treatment, rapid recovery from the acute symptoms can be expected, and the risk of coronary artery aneurysms is greatly reduced. Untreated, the acute symptoms of Kawasaki disease are self-limited (i.e. the patient will recover eventually), but the risk of coronary artery involvement is much greater, even many years later. Many cases of myocardial infarction in young adults have now been attributed to Kawasaki disease that went undiagnosed during childhood.[6] Overall, about 2% of patients die from complications of coronary vasculitis.[citation needed]
Laboratory evidence of increased inflammation combined with demographic features (male sex, age less than six months or greater than eight years) and incomplete response to IVIG therapy create a profile of a high-risk patient with Kawasaki disease.[59][153] The likelihood that an aneurysm will resolve appears to be determined in large measure by its initial size, in which the smaller aneurysms have a greater likelihood of regression.[154][155] Other factors are positively associated with the regression of aneurysms, including being younger than a year old at the onset of Kawasaki disease, fusiform rather than saccular aneurysm morphology, and an aneurysm location in a distal coronary segment.[61] The highest rate of progression to stenosis occurs among those who develop large aneurysms.[4] The worst prognosis occurs in children with giant aneurysms.[156] This severe outcome may require further treatment such as percutaneous transluminal angioplasty,[157] coronary artery stenting,[158] bypass grafting,[159] and even cardiac transplantation.[160]
A relapse of symptoms may occur soon after initial treatment with IVIG. This usually requires rehospitalization and retreatment. Treatment with IVIG can cause allergic and nonallergic acute reactions, aseptic meningitis, fluid overload, and rarely, other serious reactions. Overall, life-threatening complications resulting from therapy for Kawasaki disease are exceedingly rare, especially compared with the risk of nontreatment. Evidence indicates Kawasaki disease produces altered lipid metabolism that persists beyond the clinical resolution of the disease.[citation needed]
Rarely, recurrence can occur in Kawasaki disease with or without treatment.[161][162]
Epidemiology
[edit]Kawasaki disease affects boys more than girls, with people of Asian ethnicity, particularly Japanese people. The higher incidence in Asian populations is thought to be linked to genetic susceptibility.[163] Incidence rates vary between countries.
Currently, Kawasaki disease is the most commonly diagnosed pediatric vasculitis in the world. By far, the highest incidence of Kawasaki disease occurs in Japan, with the most recent study placing the attack rate at 218.6 per 100,000 children less than five years of age (about one in 450 children). At this present attack rate, more than one in 150 children in Japan will develop Kawasaki disease during their lifetimes.[citation needed]
However, its incidence in the United States is increasing. Kawasaki disease is predominantly a disease of young children, with 80% of patients younger than five years of age. About 2,000–4,000 cases are identified in the U.S. each year (9 to 19 per 100,000 children younger than five years of age).[140][164][165] In the continental United States, Kawasaki disease is more common during the winter and early spring, boys with the disease outnumber girls by ≈1.5–1.7:1, and 76% of affected children are less than 5 years of age.[166]
In the United Kingdom, prior to 2000, it was diagnosed in fewer than one in every 25,000 people per year.[167] Incidence of the disease doubled from 1991 to 2000, however, with four cases per 100,000 children in 1991 compared with a rise of eight cases per 100,000 in 2000.[168] By 2017, this figure had risen to 12 in 100,000 people with 419 diagnosed cases of Kawasaki disease in the United Kingdom.[169]
In Japan, the rate is 240 in every 100,000 people.[170]
Coronary artery aneurysms due to Kawasaki disease are believed to account for 5% of acute coronary syndrome cases in adults under 40 years of age.[6]
History
[edit]The disease was first reported by Tomisaku Kawasaki in a four-year-old child with a rash and fever at the Red Cross Hospital in Tokyo in January 1961, and he later published a report on 50 similar cases.[15] Later, Kawasaki and colleagues were persuaded of definite cardiac involvement when they studied and reported 23 cases, of which 11 (48%) patients had abnormalities detected by an electrocardiogram.[171] In 1974, the first description of this disorder was published in the English-language literature.[172] In 1976, Melish et al. described the same illness in 16 children in Hawaii.[173] Melish and Kawasaki had independently developed the same diagnostic criteria for the disorder, which are still used today to make the diagnosis of classic Kawasaki disease.
A question was raised whether the disease only started during the period between 1960 and 1970, but later a preserved heart of a seven-year-old boy who died in 1870 was examined and showed three aneurysms of the coronary arteries with clots, as well as pathologic changes consistent with Kawasaki disease.[174] Kawasaki disease is now recognized worldwide. Why cases began to emerge across all continents around the 1960s and 1970s is unclear.[175] Possible explanations could include confusion with other diseases such as scarlet fever, and easier recognition stemming from modern healthcare factors such as the widespread use of antibiotics.[175] In particular, old pathological descriptions from Western countries of infantile polyarteritis nodosa coincide with reports of fatal cases of Kawasaki disease.[6]
In the United States and other developed nations, Kawasaki disease appears to have replaced acute rheumatic fever as the most common cause of acquired heart disease in children.[176]
References
[edit]- ^ a b c d e f g h i j k l m n o p q r s t u "Kawasaki Disease". PubMed Health. NHLBI Health Topics. 11 June 2014. Archived from the original on 11 September 2017. Retrieved 26 August 2016.
- ^ Rapini, Ronald P.; Bolognia, Jean L.; Jorizzo, Joseph L. (2007). Dermatology: 2-Volume Set. St. Louis: Mosby. pp. 1232–34. ISBN 978-1-4160-2999-1.
- ^ a b c Guidance – Paediatric multisystem inflammatory syndrome temporally associated with COVID-19 (PDF), The Royal College of Paediatrics and Child Health, 2020
- ^ a b c d e f g h i j k l m Kim DS (December 2006). "Kawasaki disease". Yonsei Medical Journal. 47 (6): 759–72. doi:10.3349/ymj.2006.47.6.759. PMC 2687814. PMID 17191303.
- ^ a b c Lai WW, Mertens LL, Cohen MS, Geva T (2015). Echocardiography in Pediatric and Congenital Heart Disease: From Fetus to Adult (2 ed.). John Wiley & Sons. p. 739. ISBN 9781118742488. Archived from the original on 11 September 2017.
- ^ a b c d e f g h i j k l m n o McCrindle BW, Rowley AH, Newburger JW, Burns JC, Bolger AF, Gewitz M, Baker AL, Jackson MA, Takahashi M, Shah PB, Kobayashi T, Wu MH, Saji TT, Pahl E (2017). "Diagnosis, Treatment, and Long-Term Management of Kawasaki Disease: A Scientific Statement for Health Professionals From the American Heart Association". Circulation. 135 (17): e927 – e999. doi:10.1161/CIR.0000000000000484. PMID 28356445."Correction". Circulation. 140 (5): e181 – e184. 2019. doi:10.1161/CIR.0000000000000703. PMID 31356128.
- ^ Owens, AM (2023). Kawasaki Disease. StatPearls Publishing.
- ^ a b c Modesti, AM; Plewa, MC (24 July 2019). "Kawasaki Disease". StatPearls. StatPearls Publishing. PMID 30725848. Archived from the original on 6 May 2020.
- ^ a b c de Graeff N, Groot N, Ozen S, et al. (2019). "European consensus-based recommendations for the diagnosis and treatment of Kawasaki disease – the SHARE initiative" (PDF). Rheumatology (Oxford). 58 (4): 672–82. doi:10.1093/rheumatology/key344. PMID 30535127. S2CID 54477877.
- ^ a b Galeotti C, Bayry J (2020). "Autoimmune and inflammatory diseases following COVID-19". Nature Reviews. Rheumatology. 16 (8): 413–414. doi:10.1038/s41584-020-0448-7. PMC 7271827. PMID 32499548.
- ^ Lin J, Harahsheh AS, Raghuveer G, et al. (2023). "Emerging Insights Into the Pathophysiology of Multisystem Inflammatory Syndrome Associated With COVID-19 in Children". Can J Cardiol. 39 (6): 793–802. doi:10.1016/j.cjca.2023.01.002. PMC 9824951. PMID 36626979.
- ^ Abrams JY, Godfred-Cato SE, Oster ME, et al. (2020). "Multisystem inflammatory syndrome in children (MIS-C) associated with SARS-CoV-2: a systematic review". The Journal of Pediatrics. 226: 45–54.e1. doi:10.1016/j.jpeds.2020.08.003. PMC 7403869. PMID 32768466.
- ^ a b "Merck Manual, Online edition: Kawasaki Disease". 2014. Archived from the original on 2 January 2010. Retrieved 26 August 2016.
- ^ a b c Brogan P, Burns JC, Cornish J, et al. (2020). "Lifetime cardiovascular management of patients with previous Kawasaki disease". Heart. 106 (6): 411–20. doi:10.1136/heartjnl-2019-315925. PMC 7057818. PMID 31843876.
- ^ a b Kawasaki T (March 1967). "[Acute febrile mucocutaneous syndrome with lymphoid involvement with specific desquamation of the fingers and toes in children]". Arerugi. 16 (3): 178–222. PMID 6062087.
- ^ a b c d e f g h i j k l m n o p q Rowley AH, Shulman ST (July 1998). "Kawasaki syndrome". Clinical Microbiology Reviews. 11 (3): 405–14. doi:10.1128/CMR.11.3.405. PMC 88887. PMID 9665974.
- ^ a b Kawasaki T (January 1995). "General review and problems in Kawasaki disease". Japanese Heart Journal. 36 (1): 1–12. doi:10.1536/ihj.36.1. PMID 7760506.
- ^ Cassidy JT, Petty RE (1995). "Vasculitis". Textbook of pediatric rheumatology (3rd ed.). Saunders. pp. 365–422. ISBN 0721652441.
- ^ Fukushige J, Takahashi N, Ueda Y, Ueda K (October 1994). "Incidence and clinical features of incomplete Kawasaki disease". Acta Paediatrica. 83 (10): 1057–60. doi:10.1111/j.1651-2227.1994.tb12985.x. PMID 7841704. S2CID 40359434.
- ^ Rowley AH, Gonzalez-Crussi F, Gidding SS, Duffy CE, Shulman ST (March 1987). "Incomplete Kawasaki disease with coronary artery involvement". The Journal of Pediatrics. 110 (3): 409–13. doi:10.1016/S0022-3476(87)80503-6. PMID 3819942.
- ^ Rodriguez-Lozano AL, Rivas-Larrauri FE, Hernandez-Bautista VM, Yamazaki-Nakashimada MA (September 2012). "Fever is not always present in Kawasaki disease". Rheumatology International. 32 (9): 2953–54. doi:10.1007/s00296-011-2123-4. PMID 21881982. S2CID 1471650.
- ^ a b Mori M, Imagawa T, Yasui K, Kanaya A, Yokota S (August 2000). "Predictors of coronary artery lesions after intravenous gamma-globulin treatment in Kawasaki disease". The Journal of Pediatrics. 137 (2): 177–80. doi:10.1067/mpd.2000.107890. PMID 10931408.
- ^ Newburger JW, Takahashi M, Beiser AS, Burns JC, Bastian J, Chung KJ, et al. (June 1991). "A single intravenous infusion of gamma globulin as compared with four infusions in the treatment of acute Kawasaki syndrome". The New England Journal of Medicine. 324 (23): 1633–9. doi:10.1056/NEJM199106063242305. PMID 1709446.
- ^ a b c d e f Yun SH, Yang NR, Park SA (July 2011). "Associated symptoms of kawasaki disease". Korean Circulation Journal. 41 (7): 394–8. doi:10.4070/kcj.2011.41.7.394. PMC 3152734. PMID 21860641.
- ^ Martínez Ruiz M, del Castillo Martín F, Borque Andrés C, García Miguel MJ, de José Gómez MI, Martínez Cortés F, Baquero Artigao F (October 2003). "Incidencia y características clínicas de la enfermedad de Kawasaki" [Incidence and clinical characteristics of Kawasaki's disease]. Anales de Pediatria (in Spanish). 59 (4): 323–27. doi:10.1016/S1695-4033(03)78190-9. PMID 14519302.
- ^ Svobodová D, Slaný J, Pískovský T (2008). "[Kawasaki disease and its ocular manifestations]" [Kawasaki disease and its ocular manifestations]. Casopis Lekaru Ceskych (in Czech). 147 (3): 162–4. PMID 18401983.
- ^ Burns JC, Joffe L, Sargent RA, Glode MP (1985). "Anterior uveitis associated with Kawasaki syndrome". Pediatric Infectious Disease. 4 (3): 258–261. doi:10.1097/00006454-198505000-00010. PMID 4039819. S2CID 40875550.
- ^ Bachmeyer C, Turc Y, Curan D, Duval-Arnould M (January 2000). "Anterior uveitis as the initial sign of adult Kawasaki syndrome (mucocutaneous lymph node syndrome)". American Journal of Ophthalmology. 129 (1): 101–2. doi:10.1016/S0002-9394(99)00285-8. PMID 10653425.
- ^ Smith LB, Newburger JW, Burns JC (February 1989). "Kawasaki syndrome and the eye". The Pediatric Infectious Disease Journal. 8 (2): 116–8. PMID 2468129.
- ^ a b Kubota M, Usami I, Yamakawa M, Tomita Y, Haruta T (June 2008). "Kawasaki disease with lymphadenopathy and fever as sole initial manifestations". Journal of Paediatrics and Child Health. 44 (6): 359–62. doi:10.1111/j.1440-1754.2008.01310.x. PMID 18476929. S2CID 32280647.
- ^ a b Scardina GA, Fucà G, Carini F, Valenza V, Spicola M, Procaccianti P, et al. (December 2007). "Oral necrotizing microvasculitis in a patient affected by Kawasaki disease" (PDF). Medicina Oral, Patologia Oral y Cirugia Bucal. 12 (8): E560–4. PMID 18059239.
- ^ a b c d e f g h i j k l m Castro PA, Urbano LM, Costa IM (August 2009). "[Kawasaki disease]". Anais Brasileiros de Dermatologia (in Portuguese). 84 (4): 317–29. doi:10.1590/S0365-05962009000400002. PMID 19851663.
- ^ Stamos JK, Corydon K, Donaldson J, Shulman ST (March 1994). "Lymphadenitis as the dominant manifestation of Kawasaki disease". Pediatrics. 93 (3): 525–8. doi:10.1542/peds.93.3.525. PMID 8115224. S2CID 32616639.
- ^ Suddleson EA, Reid B, Woolley MM, Takahashi M (October 1987). "Hydrops of the gallbladder associated with Kawasaki syndrome" (PDF). Journal of Pediatric Surgery. 22 (10): 956–9. doi:10.1016/S0022-3468(87)80600-0. PMID 3316594.
- ^ Do HJ, Baek JG, Kim HJ, Yeom JS, Park JS, Park ES, et al. (November 2009). "Kawasaki disease presenting as parotitis in a 3-month-old infant". Korean Circulation Journal. 39 (11): 502–4. doi:10.4070/kcj.2009.39.11.502. PMC 2790127. PMID 19997548.
- ^ Castro, P. A.; Urbano, L. M.; Costa, I. M. (2009). "Quadro/Chart 3: Secondary clinical findings of Kawasaki disease". Anais Brasileiros de Dermatologia. 84 (4): 317–29. doi:10.1590/S0365-05962009000400002. PMID 19851663.
- ^ Wang S, Best BM, Burns JC (June 2009). "Periungual desquamation in patients with Kawasaki disease". The Pediatric Infectious Disease Journal. 28 (6): 538–9. doi:10.1097/INF.0b013e3181945984. PMC 2738931. PMID 19483521.
- ^ Michie C, Kinsler V, Tulloh R, Davidson S (October 2000). "Recurrent skin peeling following Kawasaki disease". Archives of Disease in Childhood. 83 (4): 353–5. doi:10.1136/adc.83.4.353. PMC 1718513. PMID 10999876.
- ^ a b López Neyra A, Alvarez-Coca González J, Pérez Suárez E, Martínez Pérez J, Rubio Villanueva JL (December 2007). "Líneas de Beau y enfermedad de Kawasaki" [Beau's lines and Kawasaki disease]. Anales de Pediatria (in Spanish). 67 (6): 610–1. doi:10.1016/s1695-4033(07)70817-2. PMID 18053534.
- ^ González Pascual E, Villanueva Lamas J, Ros Viladoms J, Pons Odena M, Ruiz García-Diego S (January 1999). "Enfermedad de Kawasaki: presentación de cincuenta casos" [Kawasaki disease: A report of 50 cases] (PDF). Anales Espanoles de Pediatria (in Spanish). 50 (1): 39–43. PMID 10083641.
- ^ Kwan YW, Leung CW (December 2005). "Pustulo-vesicular skin eruption in a child with probable Kawasaki disease". European Journal of Pediatrics. 164 (12): 770–1. doi:10.1007/s00431-005-1715-y. PMID 16010565. S2CID 22194695.
- ^ Ulloa-Gutierrez R, Acón-Rojas F, Camacho-Badilla K, Soriano-Fallas A (December 2007). "Pustular rash in Kawasaki syndrome". The Pediatric Infectious Disease Journal. 26 (12): 1163–5. doi:10.1097/INF.0b013e31814619ec. PMID 18043462.
- ^ Dajani AS, Taubert KA, Gerber MA, Shulman ST, Ferrieri P, Freed M, et al. (May 1993). "Diagnosis and therapy of Kawasaki disease in children". Circulation. 87 (5): 1776–80. doi:10.1161/01.CIR.87.5.1776. PMID 8491037.
- ^ a b c Fujiwara H, Fujiwara T, Kao TC, Ohshio G, Hamashima Y (June 1986). "Pathology of Kawasaki disease in the healed stage. Relationships between typical and atypical cases of Kawasaki disease". Pathology International. 36 (6): 857–67. doi:10.1111/j.1440-1827.1986.tb03119.x. PMID 3766134. S2CID 12989507.
- ^ Dahdah N (April 2010). "Not just coronary arteritis, Kawasaki disease is a myocarditis, too". Journal of the American College of Cardiology. 55 (14): 1507, author reply 1507–8. doi:10.1016/j.jacc.2009.11.067. PMID 20359606.
- ^ Tse SM, Silverman ED, McCrindle BW, Yeung RS (April 2002). "Early treatment with intravenous immunoglobulin in patients with Kawasaki disease". The Journal of Pediatrics. 140 (4): 450–5. doi:10.1067/mpd.2002.122469. PMID 12006960.
- ^ "Clinical manifestations of Kawasaki disease". Archived from the original on 20 April 2012. Retrieved 1 December 2011.[non-primary source needed][full citation needed]
- ^ a b c Marchesi A, Tarissi de Jacobis I, Rigante D, Rimini A, Malorni W, et al. (2018). "Kawasaki disease: guidelines of the Italian Society of Pediatrics, part I – definition, epidemiology, etiopathogenesis, clinical expression and management of the acute phase". Italian Journal of Pediatrics. 44 (1): 102. doi:10.1186/s13052-018-0536-3. PMC 6116535. PMID 30157897.
- ^ a b Hirose O, Misawa H, Kijima Y, Yamada O, Arakaki Y, Kajino Y, et al. (March 1981). "[Two-dimensional echocardiography of coronary artery in Kawasaki disease (MCLS): detection, changes in acute phase, and follow-up observation of the aneurysm (author's transl)]". Journal of Cardiography (in Japanese). 11 (1): 89–104. PMID 7264399.
- ^ a b Wolff AE, Hansen KE, Zakowski L (May 2007). "Acute Kawasaki disease: not just for kids". Journal of General Internal Medicine. 22 (5): 681–4. doi:10.1007/s11606-006-0100-5. PMC 1852903. PMID 17443379.
- ^ Burns JC, Wiggins JW, Toews WH, Newburger JW, Leung DY, Wilson H, Glodé MP (November 1986). "Clinical spectrum of Kawasaki disease in infants younger than 6 months of age". The Journal of Pediatrics. 109 (5): 759–63. doi:10.1016/S0022-3476(86)80689-8. PMID 3772656.
- ^ Boven K, De Graeff-Meeder ER, Spliet W, Kuis W (August 1992). "Atypical Kawasaki disease: an often missed diagnosis". European Journal of Pediatrics. 151 (8): 577–80. doi:10.1007/BF01957725. PMID 1505575. S2CID 6125622.
- ^ Suzuki A, Kamiya T, Kuwahara N, Ono Y, Kohata T, Takahashi O, et al. (1986). "Coronary arterial lesions of Kawasaki disease: cardiac catheterization findings of 1100 cases". Pediatric Cardiology. 7 (1): 3–9. doi:10.1007/BF02315475. PMID 3774580. S2CID 20301847.
- ^ Durongpisitkul K, Gururaj VJ, Park JM, Martin CF (December 1995). "The prevention of coronary artery aneurysm in Kawasaki disease: a meta-analysis on the efficacy of aspirin and immunoglobulin treatment". Pediatrics. 96 (6): 1057–61. doi:10.1542/peds.96.6.1057. PMID 7491221. S2CID 22210926. Archived from the original on 2 May 2020.
- ^ Terai M, Shulman ST (December 1997). "Prevalence of coronary artery abnormalities in Kawasaki disease is highly dependent on gamma globulin dose but independent of salicylate dose". The Journal of Pediatrics. 131 (6): 888–93. doi:10.1016/S0022-3476(97)70038-6. PMID 9427895.
- ^ Dajani AS, Taubert KA, Takahashi M, Bierman FZ, Freed MD, Ferrieri P, et al. (February 1994). "Guidelines for long-term management of patients with Kawasaki disease. Report from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association". Circulation. 89 (2): 916–22. doi:10.1161/01.cir.89.2.916. PMID 8313588.
- ^ Kobayashi T, Inoue Y, Morikawa A (February 2008). "[Risk stratification and prediction of resistance to intravenous immunoglobulin in Kawasaki disease]". Nihon Rinsho. Japanese Journal of Clinical Medicine (in Japanese). 66 (2): 332–7. PMID 18260333.
- ^ Harada K (December 1991). "Intravenous gamma-globulin treatment in Kawasaki disease". Acta Paediatrica Japonica. 33 (6): 805–10. doi:10.1111/j.1442-200X.1991.tb02612.x. PMID 1801561. S2CID 56991692.
- ^ a b Koren G, Lavi S, Rose V, Rowe R (March 1986). "Kawasaki disease: review of risk factors for coronary aneurysms". The Journal of Pediatrics. 108 (3): 388–92. doi:10.1016/S0022-3476(86)80878-2. PMID 3950818.
- ^ a b c d Kato H, Sugimura T, Akagi T, Sato N, Hashino K, Maeno Y, et al. (September 1996). "Long-term consequences of Kawasaki disease. A 10- to 21-year follow-up study of 594 patients". Circulation. 94 (6): 1379–85. doi:10.1161/01.cir.94.6.1379. PMID 8822996.
- ^ a b Takahashi M, Mason W, Lewis AB (February 1987). "Regression of coronary aneurysms in patients with Kawasaki syndrome". Circulation. 75 (2): 387–94. doi:10.1161/01.CIR.75.2.387. PMID 3802442.
- ^ a b c Kato H, Ichinose E, Kawasaki T (June 1986). "Myocardial infarction in Kawasaki disease: clinical analyses in 195 cases". The Journal of Pediatrics. 108 (6): 923–7. doi:10.1016/S0022-3476(86)80928-3. PMID 3712157.
- ^ Suzuki A, Kamiya T, Tsuchiya K, Sato I, Arakaki Y, Kohata T, Ono Y (February 1988). "Tricuspid and mitral regurgitation detected by color flow Doppler in the acute phase of Kawasaki disease". The American Journal of Cardiology. 61 (4): 386–90. doi:10.1016/0002-9149(88)90950-2. PMID 3341217.
- ^ Akagi T, Kato H, Inoue O, Sato N, Imamura K (August 1990). "Valvular heart disease in Kawasaki syndrome: incidence and natural history". American Heart Journal. 120 (2): 366–72. doi:10.1016/0002-8703(90)90081-8. PMID 2382613.
- ^ Gidding SS, Shulman ST, Ilbawi M, Crussi F, Duffy CE (April 1986). "Mucocutaneous lymph node syndrome (Kawasaki disease): delayed aortic and mitral insufficiency secondary to active valvulitis". Journal of the American College of Cardiology. 7 (4): 894–7. doi:10.1016/S0735-1097(86)80354-0. PMID 3958349.
- ^ Fukunaga S, Egashira A, Arinaga K, Akasu I, Kai E, Higashi T, et al. (March 1996). "Aortic valve replacement for aortic regurgitation due to Kawasaki disease. Report of two cases". The Journal of Heart Valve Disease. 5 (2): 231–4. PMID 8665019.
- ^ Ravekes WJ, Colan SD, Gauvreau K, Baker AL, Sundel RP, van der Velde ME, et al. (April 2001). "Aortic root dilation in Kawasaki disease". The American Journal of Cardiology. 87 (7): 919–22. doi:10.1016/S0002-9149(00)01541-1. PMID 11274955.
- ^ Fuyama Y, Hamada R, Uehara R, Yano I, Fujiwara M, Matoba M, et al. (June 1996). "Long-term follow up of abdominal aortic aneurysm complicating Kawasaki disease: comparison of the effectiveness of different imaging methods". Acta Paediatrica Japonica. 38 (3): 252–5. doi:10.1111/j.1442-200X.1996.tb03480.x. PMID 8741316. S2CID 30968335.
- ^ Miyake T, Yokoyama T, Shinohara T, Seto S, Oiki M (August 1995). "Transient dilatation of the abdominal aorta in an infant with Kawasaki disease associated with thrombocytopenia". Acta Paediatrica Japonica. 37 (4): 521–5. doi:10.1111/j.1442-200X.1995.tb03368.x. PMID 7572158. S2CID 30747089.
- ^ a b c d e Alves NR, Magalhães CM, Almeida R, Santos RC, Gandolfi L, Pratesi R (June 2011). "Prospective study of Kawasaki disease complications: review of 115 cases". Revista da Associação Médica Brasileira. 57 (3): 295–300. doi:10.1016/s2255-4823(11)70062-5. PMID 21691693.
- ^ Yang G, Thompson D, Warren A (February 2009). "Late-appearing brachiocephalic aneurysm: an atypical vascular sequella of Kawasaki disease". Pediatric Cardiology. 30 (2): 197–9. doi:10.1007/s00246-008-9296-y. PMID 18704549. S2CID 11627024.
- ^ a b Dhillon R, Clarkson P, Donald AE, Powe AJ, Nash M, Novelli V, et al. (November 1996). "Endothelial dysfunction late after Kawasaki disease". Circulation. 94 (9): 2103–6. doi:10.1161/01.cir.94.9.2103. PMID 8901658.
- ^ Cheung YF, Wong SJ, Ho MH (January 2007). "Relationship between carotid intima-media thickness and arterial stiffness in children after Kawasaki disease". Archives of Disease in Childhood. 92 (1): 43–47. doi:10.1136/adc.2006.096628. PMC 2083125. PMID 16820386.
- ^ Ooyanagi R, Fuse S, Tomita H, Takamuro M, Horita N, Mori M, Tsutsumi H (August 2004). "Pulse wave velocity and ankle brachial index in patients with Kawasaki disease". Pediatrics International. 46 (4): 398–402. doi:10.1111/j.1442-200x.2004.01929.x. PMID 15310302. S2CID 21586626.
- ^ a b Cheung YF, Yung TC, Tam SC, Ho MH, Chau AK (January 2004). "Novel and traditional cardiovascular risk factors in children after Kawasaki disease: implications for premature atherosclerosis". Journal of the American College of Cardiology. 43 (1): 120–4. doi:10.1016/j.jacc.2003.08.030. PMID 14715193.
- ^ Yaniv L, Jaffe M, Shaoul R (September 2005). "The surgical manifestations of the intestinal tract in Kawasaki disease". Journal of Pediatric Surgery. 40 (9): e1–4. doi:10.1016/j.jpedsurg.2005.05.063. PMID 16150324.
- ^ Kim MY, Noh JH (August 2008). "A case of Kawasaki disease with colonic edema". Journal of Korean Medical Science. 23 (4): 723–6. doi:10.3346/jkms.2008.23.4.723. PMC 2526417. PMID 18756065.
- ^ Beiler HA, Schmidt KG, von Herbay A, Löffler W, Daum R (April 2001). "Ischemic small bowel strictures in a case of incomplete Kawasaki disease". Journal of Pediatric Surgery. 36 (4): 648–50. doi:10.1053/jpsu.2001.22311. PMID 11283899.
- ^ Akikusa JD, Laxer RM, Friedman JN (May 2004). "Intestinal pseudoobstruction in Kawasaki disease". Pediatrics. 113 (5): e504–6. doi:10.1542/peds.113.5.e504. PMID 15121996.
- ^ Zulian F, Falcini F, Zancan L, Martini G, Secchieri S, Luzzatto C, Zacchello F (June 2003). "Acute surgical abdomen as presenting manifestation of Kawasaki disease". The Journal of Pediatrics. 142 (6): 731–5. doi:10.1067/mpd.2003.232. PMID 12838207.
- ^ Ohno S, Miyajima T, Higuchi M, Yoshida A, Matsuda H, Saheki Y, et al. (June 1982). "Ocular manifestations of Kawasaki's disease (mucocutaneous lymph node syndrome)". American Journal of Ophthalmology. 93 (6): 713–7. doi:10.1016/0002-9394(82)90465-2. PMID 7201245.
- ^ Burke MJ, Rennebohm RM (1981). "Eye involvement in Kawasaki disease". Journal of Pediatric Ophthalmology and Strabismus. 18 (5): 7–11. doi:10.3928/0191-3913-19810901-04. PMID 7299613.
- ^ Anand S, Yang YC (2004). "Optic disc changes in Kawasaki disease". Journal of Pediatric Ophthalmology and Strabismus. 41 (3): 177–9. doi:10.3928/0191-3913-20040501-12. PMID 15206604.
- ^ Farvardin M, Kashef S, Aleyasin S, Nabavizadeh SH, Sajjadi M, Safari M (2007). "Sudden unilateral blindness in a girl with Kawasaki disease". Journal of Pediatric Ophthalmology and Strabismus. 44 (5): 303–04. doi:10.3928/01913913-20070901-06. PMID 17913174.
- ^ Tomita S, Chung K, Mas M, Gidding S, Shulman ST (January 1992). "Peripheral gangrene associated with Kawasaki disease". Clinical Infectious Diseases. 14 (1): 121–6. doi:10.1093/clinids/14.1.121. PMID 1571415.
- ^ Tabarki B, Mahdhaoui A, Selmi H, Yacoub M, Essoussi AS (September 2001). "Kawasaki disease with predominant central nervous system involvement". Pediatric Neurology. 25 (3): 239–41. doi:10.1016/S0887-8994(01)00290-9. PMID 11587880.
- ^ Takagi K, Umezawa T, Saji T, Morooka K, Matsuo N (September 1990). "[Meningoencephalitis in Kawasaki disease]". No to Hattatsu = Brain and Development (in Japanese). 22 (5): 429–35. PMID 2223179.
- ^ Aoki N (March 1988). "Subdural effusion in the acute stage of Kawasaki disease (Mucocutaneous lymph node syndrome)". Surgical Neurology. 29 (3): 216–7. doi:10.1016/0090-3019(88)90009-2. PMID 3344468.
- ^ Bailie NM, Hensey OJ, Ryan S, Allcut D, King MD (2001). "Bilateral subdural collections--an unusual feature of possible Kawasaki disease". European Journal of Paediatric Neurology. 5 (2): 79–81. doi:10.1053/ejpn.2001.0469. PMID 11589317.
- ^ a b Ichiyama T, Nishikawa M, Hayashi T, Koga M, Tashiro N, Furukawa S (July 1998). "Cerebral hypoperfusion during acute Kawasaki disease". Stroke. 29 (7): 1320–1. doi:10.1161/01.STR.29.7.1320. PMID 9660380.
- ^ a b Fujiwara S, Yamano T, Hattori M, Fujiseki Y, Shimada M (1992). "Asymptomatic cerebral infarction in Kawasaki disease". Pediatric Neurology. 8 (3): 235–6. doi:10.1016/0887-8994(92)90077-C. PMID 1622525.
- ^ Muneuchi J, Kusuhara K, Kanaya Y, Ohno T, Furuno K, Kira R, et al. (January 2006). "Magnetic resonance studies of brain lesions in patients with Kawasaki disease". Brain & Development. 28 (1): 30–33. doi:10.1016/j.braindev.2005.04.003. PMID 15967620. S2CID 19461613.
- ^ Wright H, Waddington C, Geddes J, Newburger JW, Burgner D (September 2008). "Facial nerve palsy complicating Kawasaki disease". Pediatrics. 122 (3): e783–5. doi:10.1542/peds.2007-3238. PMID 18678602. S2CID 207161115.
- ^ Knott PD, Orloff LA, Harris JP, Novak RE, Burns JC (2001). "Sensorineural hearing loss and Kawasaki disease: a prospective study" (PDF). American Journal of Otolaryngology. 22 (5): 343–8. doi:10.1053/ajot.2001.26495. PMID 11562886.
- ^ Silva CH, Roscoe IC, Fernandes KP, Novaes RM, Lázari CS (2002). "[Sensorineural hearing loss associated to Kawasaki Disease]". Jornal de Pediatria (in English and Portuguese). 78 (1): 71–74. doi:10.2223/JPED.669. PMID 14647816.
- ^ Carlton-Conway D, Ahluwalia R, Henry L, Michie C, Wood L, Tulloh R (May 2005). "Behaviour sequelae following acute Kawasaki disease". BMC Pediatrics. 5 (1): 14. doi:10.1186/1471-2431-5-14. PMC 1156909. PMID 15916701.
- ^ King WJ, Schlieper A, Birdi N, Cappelli M, Korneluk Y, Rowe PC (May 2000). "The effect of Kawasaki disease on cognition and behavior". Archives of Pediatrics & Adolescent Medicine. 154 (5): 463–8. doi:10.1001/archpedi.154.5.463. PMID 10807296.
- ^ Rowley AH, Baker SC, Orenstein JM, Shulman ST (May 2008). "Searching for the cause of Kawasaki disease-cytoplasmic inclusion bodies provide new insight". Nature Reviews. Microbiology. 6 (5): 394–401. doi:10.1038/nrmicro1853. PMC 7097362. PMID 18364728.
- ^ "Kawasaki Disease". American Heart Association. Archived from the original on 29 December 2008. Retrieved 3 January 2009.
- ^ "Kawasaki Disease: Causes". Mayo Clinic. Archived from the original on 12 December 2008. Retrieved 3 January 2009.
- ^ a b c d e Marrani E, Burns JC, Cimaz R (2018). "How Should We Classify Kawasaki Disease?". Frontiers in Immunology. 9: 2974. doi:10.3389/fimmu.2018.02974. PMC 6302019. PMID 30619331.
- ^ Rowley AH, Shulman ST (2018). "The Epidemiology and Pathogenesis of Kawasaki Disease". Frontiers in Pediatrics. 6: 374. doi:10.3389/fped.2018.00374. PMC 6298241. PMID 30619784.
- ^ a b Lo MS (2020). "A framework for understanding Kawasaki disease pathogenesis". Clinical Immunology (Orlando, Fla.). 214: 108385. doi:10.1016/j.clim.2020.108385. PMID 32173601.
- ^ Nakamura Y, Yashiro M, Uehara R, Oki I, Watanabe M, Yanagawa H (2008). "Monthly observation of the number of patients with Kawasaki disease and its incidence rates in Japan: chronological and geographical observation from nationwide surveys". Journal of Epidemiology. 18 (6): 273–9. doi:10.2188/jea.JE2008030. PMC 4771612. PMID 19075496.
- ^ Rowley AH (2018). "Is Kawasaki disease an infectious disorder?". International Journal of Rheumatic Diseases. 21 (1): 20–25. doi:10.1111/1756-185X.13213. PMC 5777874. PMID 29105346.
- ^ Freeman AF, Shulman ST (June 2001). "Recent developments in Kawasaki disease". Current Opinion in Infectious Diseases. 14 (3): 357–61. doi:10.1097/00001432-200106000-00017. PMID 11964855. S2CID 24155085.
- ^ Rowley AH (June 2020). "Understanding SARS-CoV-2-related multisystem inflammatory syndrome in children". Nature Reviews. Immunology. 20 (8): 453–4. doi:10.1038/s41577-020-0367-5. PMC 7296515. PMID 32546853.
- ^ Soni PR, Noval Rivas M, Arditi M (2020). "A Comprehensive Update on Kawasaki Disease Vasculitis and Myocarditis". Current Rheumatology Reports. 22 (2): 6. doi:10.1007/s11926-020-0882-1. PMID 32020498. S2CID 211034768.
- ^ Rodó X, Ballester J, Cayan D, Melish ME, Nakamura Y, Uehara R, Burns JC (10 November 2011). "Association of Kawasaki disease with tropospheric wind patterns". Scientific Reports. 1: 152. Bibcode:2011NatSR...1E.152R. doi:10.1038/srep00152. PMC 3240972. PMID 22355668.
- ^ Ballester J, Burns JC, Cayan D, Nakamura Y, Uehara R, Rodó X (2013). "Kawasaki disease and ENSO-driven wind circulation" (PDF). Geophysical Research Letters. 40 (10): 2284–9. Bibcode:2013GeoRL..40.2284B. doi:10.1002/grl.50388.
- ^ Frazer J (April 2012). "Infectious disease: Blowing in the wind". Nature. 484 (7392): 21–23. Bibcode:2012Natur.484...21F. doi:10.1038/484021a. PMID 22481336.
- ^ Rodó, X; Curcoll, R; Robinson, M; Ballester, J; Burns, JC; Cayan, DR; Lipkin, WI; Williams, BL; Couto-Rodriguez, M; Nakamura, Y; Uehara, R; Tanimoto, H; Morguí, JA (3 June 2014). "Tropospheric winds from northeastern China carry the etiologic agent of Kawasaki disease from its source to Japan". Proceedings of the National Academy of Sciences of the United States of America. 111 (22): 7952–7. Bibcode:2014PNAS..111.7952R. doi:10.1073/pnas.1400380111. PMC 4050536. PMID 24843117.
- ^ a b Dietz SM, van Stijn D, Burgner D, et al. (2017). "Dissecting Kawasaki disease: a state-of-the-art review". European Journal of Pediatrics. 176 (8): 995–1009. doi:10.1007/s00431-017-2937-5. PMC 5511310. PMID 28656474.
- ^ a b Son, MB; Sundel, RP (2016). "Kawasaki Disease". Textbook of Pediatric Rheumatology. pp. 467–483.e6. doi:10.1016/B978-0-323-24145-8.00035-1. ISBN 9780323241458. PMC 7161397.
- ^ a b c d Elakabawi K, Lin J, Jiao F, Guo N, Yuan Z (2020). "Kawasaki Disease: Global Burden and Genetic Background". Cardiology Research. 11 (1): 9–14. doi:10.14740/cr993. PMC 7011927. PMID 32095191.
- ^ Galeotti C, Kaveri SV, Cimaz R, Koné-Paut I, Bayry J (2016). "Predisposing factors, pathogenesis and therapeutic intervention of Kawasaki disease". Drug Discovery Today. 21 (11): 1850–7. doi:10.1016/j.drudis.2016.08.004. PMC 7185772. PMID 27506874.
- ^ Behrman RE, Kliegman R, Marcdante K, Jenson HB (2006). Nelson essentials of pediatrics. St. Louis, Mo: Elsevier Saunders. ISBN 1-4160-0159-X.
- ^ a b c d Ozen S, Ruperto N, Dillon MJ, Bagga A, Barron K, Davin JC, et al. (July 2006). "EULAR/PReS endorsed consensus criteria for the classification of childhood vasculitides". Annals of the Rheumatic Diseases. 65 (7): 936–41. doi:10.1136/ard.2005.046300. PMC 1798210. PMID 16322081.
- ^ Newburger JW, Takahashi M, Burns JC (2016). "Kawasaki Disease". Journal of the American College of Cardiology. 67 (14): 1738–49. doi:10.1016/j.jacc.2015.12.073. PMID 27056781.
- ^ "Kawasaki disease – Diagnosis and treatment". mayoclinic.org. Retrieved 5 October 2018.
- ^ Shulman, Stanford T.; Taubert, Kathryn A. (June 1999). "Kawasaki Disease". American Family Physician. 59 (11): 3093–102, 3107–08. PMID 10392592. Archived from the original on 17 May 2008.
- ^ "Kawasaki Disease Diagnostic Criteria". Archived from the original on 7 August 2016. Retrieved 30 May 2016.
- ^ Kuo HC, Liang CD, Wang CL, Yu HR, Hwang KP, Yang KD (October 2010). "Serum albumin level predicts initial intravenous immunoglobulin treatment failure in Kawasaki disease". Acta Paediatrica. 99 (10): 1578–83. doi:10.1111/j.1651-2227.2010.01875.x. PMID 20491705. S2CID 13412642.
- ^ Phuong LK, Bonetto C, Buttery J, et al. (December 2016). "Kawasaki disease and immunisation: Standardised case definition & guidelines for data collection, analysis". Vaccine. 34 (51): 6582–96. doi:10.1016/j.vaccine.2016.09.025. PMID 27863715.
- ^ Sharma C, Ganigara M, Galeotti C, Burns J, Berganza FM, Hayes DA, Singh-Grewal D, Bharath S, Sajjan S, Bayry J (29 October 2021). "Multisystem inflammatory syndrome in children and Kawasaki disease: a critical comparison". Nature Reviews Rheumatology. 17 (12): 731–748. doi:10.1038/s41584-021-00709-9. PMC 8554518. PMID 34716418.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b "Multisystem inflammatory syndrome in children and adolescents with COVID-19". www.who.int. Retrieved 16 May 2020.
- ^ a b "Multisystem Inflammatory Syndrome in Children (MIS-C) Associated with Coronavirus Disease 2019 (COVID-19)". emergency.cdc.gov. Centers for Disease Control and Prevention. 15 May 2020. Retrieved 15 May 2020.
- ^ Elouardi, Y (7 January 2022). "COVID-19 associated Kawasaki-like multisystem inflammatory syndrome in an adult". Revista Española de Anestesiología y Reanimación (English Edition). 69 (1): 43–47. doi:10.1016/j.redare.2020.11.009. PMC 8739010. PMID 35031265.
- ^ Morris, Sapna Bamrah; Schwartz, Noah G.; Patel, Pragna; Abbo, Lilian; Beauchamps, Laura; Balan, Shuba; Lee, Ellen H.; Paneth-Pollak, Rachel; Geevarughese, Anita; Lash, Maura K.; Dorsinville, Marie S.; Ballen, Vennus; Eiras, Daniel P.; Newton-Cheh, Christopher; Smith, Emer; Robinson, Sara; Stogsdill, Patricia; Lim, Sarah; Fox, Sharon E.; Richardson, Gillian; Hand, Julie; Oliver, Nora T.; Kofman, Aaron; Bryant, Bobbi; Ende, Zachary; Datta, Deblina; Belay, Ermias; Godfred-Cato, Shana (9 October 2020). "Case Series of Multisystem Inflammatory Syndrome in Adults Associated with SARS-CoV-2 Infection — United Kingdom and United States, March–August 2020". Morbidity and Mortality Weekly Report. 69 (40): 1450–1456. doi:10.15585/mmwr.mm6940e1. PMC 7561225. PMID 33031361.
- ^ Shaigany, Sheila; Gnirke, Marlis; Guttman, Allison; Chong, Hong; Meehan, Shane; Raabe, Vanessa (10 July 2020). "An adult with Kawasaki-like multisystem inflammatory syndrome associated with COVID-19". The Lancet. 396 (10246): e8 – e10. doi:10.1016/S0140-6736(20)31526-9. PMC 7351414. PMID 32659211.
- ^ McCrindle BW, Manlhiot C (2020). "SARS-CoV-2-Related Inflammatory Multisystem Syndrome in Children: Different or Shared Etiology and Pathophysiology as Kawasaki Disease?". JAMA. 324 (3): 246–248. doi:10.1001/jama.2020.10370. PMID 32511667.
- ^ a b c d e Guillevin L, Pagnoux C (March 2008). "[Classification of systemic vasculitides]". La Revue du Praticien (in French). 58 (5): 480–86. PMID 18524103.
- ^ "necrotizing vasculitis – definition of necrotizing vasculitis". Free Online Medical Dictionary, Thesaurus and Encyclopedia. Retrieved 19 May 2010.
- ^ Dillon MJ, Eleftheriou D, Brogan PA (September 2010). "Medium-size-vessel vasculitis". Pediatric Nephrology. 25 (9): 1641–52. doi:10.1007/s00467-009-1336-1. PMC 2908435. PMID 19946711.
- ^ Rigante D (2006). "Clinical overview of vasculitic syndromes in the pediatric age". European Review for Medical and Pharmacological Sciences. 10 (6): 337–45. PMID 17274537. S2CID 15223179.
- ^ Brandt HR, Arnone M, Valente NY, Sotto MN, Criado PR (2009). "[Medium and large vessel vasculitis]". Anais Brasileiros de Dermatologia (in English and Portuguese). 84 (1): 55–67. doi:10.1590/S0365-05962009000100008. PMID 19377760.
- ^ Herlin T, Nielsen S (September 2008). "Primær vaskulitis i barnealderen – nye klassifikationskriterier" [Primary childhood vasculitis--new classification criteria]. Ugeskrift for Laeger (in Danish). 170 (36): 2784–87. PMID 18761873.
- ^ Guillevin L, Pagnoux C, Guilpain P (May 2007). "Classification des vascularites systémiques" [Classification of systemic vasculatides]. Presse Médicale (in French). 36 (5 Pt 2): 845–53. doi:10.1016/j.lpm.2007.01.035. PMID 17408915.
- ^ Jennette JC, Falk RJ (October 2000). "Do vasculitis categorization systems really matter?". Current Rheumatology Reports. 2 (5): 430–38. doi:10.1007/s11926-000-0044-4. PMID 11123094. S2CID 25260955.
- ^ a b "Who Kawasaki Disease Affects". Children's Hospital Boston. Archived from the original on 23 November 2008. Retrieved 4 January 2009.
- ^ a b Oates-Whitehead RM, Baumer JH, Haines L, Love S, Maconochie IK, Gupta A, et al. (2003). Baumer JH (ed.). "Intravenous immunoglobulin for the treatment of Kawasaki disease in children". The Cochrane Database of Systematic Reviews. 2021 (4): CD004000. doi:10.1002/14651858.CD004000. PMC 6544780. PMID 14584002.
- ^ Kuniyoshi Y, Tsujimoto Y, Banno M, Taito S, Ariie T, Takahashi N, Tokutake H, Takada T (May 2023). "Prediction Models for Intravenous Immunoglobulin Resistance in Kawasaki Disease: A Meta-analysis". Pediatrics. 151 (5): e2022059175. doi:10.1542/peds.2022-059175. PMID 37092277.
- ^ "Comparing Two Treatments for IVIG-Resistant Kawasaki Disease - Evidence Update for Clinicians". www.pcori.org. 9 May 2024. Retrieved 30 June 2024.
- ^ Hsieh KS, Weng KP, Lin CC, Huang TC, Lee CL, Huang SM (December 2004). "Treatment of acute Kawasaki disease: aspirin's role in the febrile stage revisited". Pediatrics. 114 (6): e689–93. doi:10.1542/peds.2004-1037. PMID 15545617.
- ^ Baumer JH, Love SJ, Gupta A, Haines LC, Maconochie I, Dua JS, et al. (Cochrane Vascular Group) (October 2006). "Salicylate for the treatment of Kawasaki disease in children". The Cochrane Database of Systematic Reviews. 2010 (4): CD004175. doi:10.1002/14651858.CD004175.pub2. PMC 8765111. PMID 17054199.
- ^ "Kawasaki Disease Treatment & Management". Medscape – EMedicine. Archived from the original on 3 February 2009. Retrieved 8 May 2020.
- ^ Kuo HC, Lo MH, Hsieh KS, Guo MM, Huang YH (2015). "High-Dose Aspirin is Associated with Anemia and Does Not Confer Benefit to Disease Outcomes in Kawasaki Disease". PLOS ONE. 10 (12): e0144603. Bibcode:2015PLoSO..1044603K. doi:10.1371/journal.pone.0144603. PMC 4686074. PMID 26658843.
- ^ Yamaji N, da Silva Lopes K, Shoda T, Ishitsuka K, Kobayashi T, Ota E, Mori R, et al. (Cochrane Vascular Group) (August 2019). "TNF-α blockers for the treatment of Kawasaki disease in children". The Cochrane Database of Systematic Reviews. 2019 (8): CD012448. doi:10.1002/14651858.CD012448.pub2. PMC 6953355. PMID 31425625.
- ^ Hamada H, Suzuki H, Onouchi Y, Ebata R, Terai M, Fuse S, Okajima Y, Kurotobi S, Hirai K, Soga T, Ishiguchi Y, Okuma Y, Takada N, Yanai M, Sato J, Nakayashiro M, Ayusawa M, Yamamoto E, Nomura Y, Hashimura Y, Ouchi K, Masuda H, Takatsuki S, Hirono K, Ariga T, Higaki T, Otsuki A, Terauchi M, Aoyagi R, Sato T, Fujii Y, Fujiwara T, Hanaoka H, Hata A, KAICA trial Investigators (March 2019). "Efficacy of primary treatment with immunoglobulin plus ciclosporin for prevention of coronary artery abnormalities in patients with Kawasaki disease predicted to be at increased risk of non-response to intravenous immunoglobulin (KAICA): a randomised controlled, open-label, blinded-endpoints, phase 3 trial". The Lancet. 393 (10176): 1128–1137. doi:10.1016/S0140-6736(18)32003-8. PMID 30853151. S2CID 72335365.
- ^ Sundel RP, Baker AL, Fulton DR, Newburger JW (June 2003). "Corticosteroids in the initial treatment of Kawasaki disease: report of a randomized trial". The Journal of Pediatrics. 142 (6): 611–16. doi:10.1067/mpd.2003.191. PMID 12838187.
- ^ Newburger JW, Sleeper LA, McCrindle BW, Minich LL, Gersony W, Vetter VL, Atz AM, Li JS, Takahashi M, Baker AL, Colan SD, Mitchell PD, Klein GL, Sundel RP (February 2007). "Randomized trial of pulsed corticosteroid therapy for primary treatment of Kawasaki disease". The New England Journal of Medicine. 356 (7): 663–75. doi:10.1056/NEJMoa061235. PMID 17301297.
- ^ Green, Jessica; Wardle, Andrew J.; Tulloh, Robert Mr (27 May 2022). "Corticosteroids for the treatment of Kawasaki disease in children". The Cochrane Database of Systematic Reviews. 2022 (5): CD011188. doi:10.1002/14651858.CD011188.pub3. ISSN 1469-493X. PMC 9139689. PMID 35622534.
- ^ Beiser AS, Takahashi M, Baker AL, Sundel RP, Newburger JW (May 1998). "A predictive instrument for coronary artery aneurysms in Kawasaki disease. US Multicenter Kawasaki Disease Study Group". The American Journal of Cardiology. 81 (9): 1116–20. doi:10.1016/S0002-9149(98)00116-7. PMID 9605052.
- ^ Fujiwara T, Fujiwara H, Hamashima Y (1987). "Size of coronary aneurysm as a determinant factor of the prognosis in Kawasaki disease: clinicopathologic study of coronary aneurysms". Progress in Clinical and Biological Research. 250: 519–20. PMID 3423060.
- ^ Nakano H, Ueda K, Saito A, Nojima K (November 1985). "Repeated quantitative angiograms in coronary arterial aneurysm in Kawasaki disease". The American Journal of Cardiology. 56 (13): 846–51. doi:10.1016/0002-9149(85)90767-2. PMID 4061324.
- ^ Tatara K, Kusakawa S (November 1987). "Long-term prognosis of giant coronary aneurysm in Kawasaki disease: an angiographic study". The Journal of Pediatrics. 111 (5): 705–10. doi:10.1016/S0022-3476(87)80246-9. PMID 3668739.
- ^ Ishii M, Ueno T, Akagi T, Baba K, Harada K, Hamaoka K, et al. (October 2001). "Guidelines for catheter intervention in coronary artery lesion in Kawasaki disease". Pediatrics International. 43 (5): 558–62. doi:10.1046/j.1442-200X.2001.01464.x. PMID 11737728. S2CID 39330448.
- ^ Akagi T, Ogawa S, Ino T, Iwasa M, Echigo S, Kishida K, et al. (August 2000). "Catheter interventional treatment in Kawasaki disease: A report from the Japanese Pediatric Interventional Cardiology Investigation group". The Journal of Pediatrics. 137 (2): 181–86. doi:10.1067/mpd.2000.107164. PMID 10931409.
- ^ Kitamura S (December 2002). "The role of coronary bypass operation on children with Kawasaki disease" (PDF). Coronary Artery Disease. 13 (8): 437–47. doi:10.1097/00019501-200212000-00009. PMID 12544719. S2CID 29371025. Archived from the original (PDF) on 9 August 2017.
- ^ Checchia PA, Pahl E, Shaddy RE, Shulman ST (October 1997). "Cardiac transplantation for Kawasaki disease". Pediatrics. 100 (4): 695–99. doi:10.1542/peds.100.4.695. PMID 9310527.
- ^ Saha A, Sarkar S (August 2018). "Recurrent Kawasaki Disease". Indian Journal of Pediatrics. 85 (8): 693–94. doi:10.1007/s12098-017-2567-y. PMID 29238944. S2CID 186231502.
- ^ Brasington, Richard D. (2014). Cardiovascular Rheumatic Diseases, An Issue of Rheumatic Disease Clinics, E-Book. Elsevier Health Sciences. p. 66. ISBN 9780323296991.
- ^ Onouchi Y (2018). "The genetics of Kawasaki disease" (pdf). International Journal of Rheumatic Diseases. 21 (1): 26–30. doi:10.1111/1756-185X.13218. PMID 29152908. S2CID 37677965.
- ^ "Kawasaki Disease". ucsfbenioffchildrens.org.
- ^ "Kawasaki Syndrome". CDC. Archived from the original on 12 July 2014. Retrieved 18 August 2014.
- ^ Holman, R. C. (2003). "Kawasaki Syndrome Hospitalizations in the United States, 1997 and 2000". Pediatrics. 112 (3): 495–501. doi:10.1542/peds.112.3.495. PMID 12949272.
- ^ "BBC Health: Kawasaki Disease". 31 March 2009. Archived from the original on 9 February 2011.
- ^ "Rare heart disease rate doubles". BBC. 17 June 2002. Archived from the original on 26 May 2004.
- ^ Rutter, Megan (20 April 2020). "P134 Collaborating with the National Congenital Anomaly and Rare Disease Registration Service to estimate national incidence of Kawasaki disease in England". Rheumatology. 59 (Supplement_2). doi:10.1093/rheumatology/keaa111.129.
- ^ "Kawasaki disease in kids at record high". The Japan Times. 17 March 2012. Retrieved 28 April 2020.
- ^ Yamamoto T, Oya T, Watanabe A (1968). "Clinical features of Kawasaki disease". Shonika Rinsho (in Japanese). 21: 291–97.
- ^ Kawasaki T, Kosaki F, Okawa S, Shigematsu I, Yanagawa H (September 1974). "A new infantile acute febrile mucocutaneous lymph node syndrome (MLNS) prevailing in Japan". Pediatrics. 54 (3): 271–76. doi:10.1542/peds.54.3.271. PMID 4153258. S2CID 13221240.
- ^ Melish ME, Hicks RM, Larson EJ (June 1976). "Mucocutaneous lymph node syndrome in the United States". American Journal of Diseases of Children. 130 (6): 599–607. doi:10.1001/archpedi.1976.02120070025006. PMID 7134.
- ^ Gee SJ (1871). "Cases of morbid anatomy: aneurysms of coronary arteries in a boy". St Bartholomew's Hosp Rep. 7: 141–8, See p. 148.
- ^ a b Burns JC (2018). "History of the worldwide emergence of Kawasaki disease". International Journal of Rheumatic Diseases. 21 (1): 13–15. doi:10.1111/1756-185X.13214. PMID 29152909. S2CID 34446653.
- ^ Taubert KA, Rowley AH, Shulman ST (1995). "A 10 year (1984–1993) United States hospital survey of Kawasaki disease". In Kato H (ed.). Kawasaki disease: Proceedings of the 5th International Kawasaki Disease Symposium, Fukuoka, Japan, 22–25 May 1995. Vol. 1093. Elsevier. pp. 34–38. ISBN 0444822003. ISSN 0531-5131.
